Businesses selling non-FDA-approved stem cell products grew four-fold in five years, study says

More than four times as many businesses and clinics than were identified in 2016 are selling stem cell products not approved by the U.S. Food and Drug Administration and lack convincing evidence of safety and efficacy, according to a five-year study conducted by University of California, Irvine Program in Public Health professor of health, society and behavior Leigh Turner. The analysis appears online in the journalCell Stem Cell.
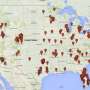
Using tools such as Google Search and Google Maps, Turner found 1,480 U.S. businesses and 2,754 clinics selling purported stem cell treatments compared to a study conducted five years ago that found 351 businesses and 570 clinics—a four-fold growth in the number of businesses operating in this space. The U.S. now has more documented facilities selling such putativestem cell treatmentsthan any other country, including nations that were once leading destinations for "stem cell tourism." California, Texas and Florida lead the nation with 347, 333, and 310 clinics respectively.
"One of the most troubling features of this marketplace is that businesses selling unproven and non-FDA-approved stem cell products often use marketing misrepresentations and aggressive sales tactics to exploit the hope, suffering, fear or desperation of patients," said Turner, corresponding author and a member of the UCI Sue & Bill Gross Stem Cell Research Center.
Stem cell therapy, or regenerative medicine, has resulted in life-saving treatment of patients with certain types of cancer and blood-related diseases, such as leukemia, lymphoma, and multiple myeloma. However, most stem cell products are, at present, investigational in nature, and their safety and efficacy continue to need testing in well-designed and rigorously conductedclinical trials.
Turner's analysis found that the types of purported treatment being offered range from claims to alleviate pain (85%+), orthopedic diseases and injuries (46%+) and sports-related injuries (22%+) all the way to hair loss and anti-aging. Some even claim to boost theimmune systemas a way to protect against the SARS CoV-2 virus.
"Many of these 'clinics' are promoting unlicensed and unproven stem cell products and claim their interventions do not require FDA approval," Turner said, who is also the ethics leader for UCI's Institute for Clinical & Translational Science. "However, that couldn't be further from the truth. I found that there is widespread promotion of products that do, in fact, require premarketing authorization by the FDA. In many cases, these clinics are using misleading advertising and predatory marketing techniques."
Additional findings show that patients are spending thousands of dollars on these unproven products. Out-of-pocket costs range anywhere from $1,200 to $28,000 with an average price tag of roughly $5,100. Some patients report suffering substantial financial losses after purchasing stem cell interventions that were allegedly promoted with misleading claims. Even more alarming is that unproven and unapproved stem cell products pose numerous risks to patients and have caused some individuals serious harm. According to the FDA, adverse events resulting from the administration of unlicensed stem cell products are likely underreported to the agency.
Turner argues that the industry has grown to a point where regulators are unable to provide adequate oversight of this marketplace. He fears that this trend seems likely to continue unless there are substantial increases in enforcement activity by the FDA, the Federal Trade Commission, and other regulatory bodies and law enforcement agencies.
"This marketplace also poses threats to the collective good by underminingpublic health, the advancement of scientific knowledge, trust inpublic institutions和所需的公众理解公民to be able to distinguish evidence-based stem cell interventions from products unsupported by convincing safety and efficacy data," Turner said.
更多的信息:Leigh Turner & colleauges, The American Stem Cell Sell in 2021: U.S. Businesses Selling Unlicensed and Unproven Stem Cell Interventions,Cell Stem Cell(2021).DOI: 10.1016/j.stem.2021.10.008.www.cell.com/cell-stem-cell/fu … 1934-5909(21)00420-3