A simple method to improve heart-attack repair using stem cell-derived heart muscle cells

The heart cannot regenerate muscle tissue after a heart attack has killed part of the muscle wall. That dead tissue can strain surrounding muscle, leading to a lethal heart enlargement.
Biomedical engineers believe they can aid the failing heart by usingpluripotent stem cellsto growheart muscle cellsoutside of the body, and then injecting thosemuscle cellsor adding a patch made from thosecells, at or near the site of the dead heart tissue. Experimental and clinical trial evidence with this approach has shown moderate improvement of the pumping ability of the heart's left ventricle.
However, the ability of the delivered cells to remuscularize the heart and improve cardiac function depends on the quality of those cells. A challenge has been low rates of engraftment by the transplanted cells.
University of Alabama at Birmingham researchers now report a simple method to improve the quality of the delivered cells, and they found that this method—tested in a mouse heart attack model—doubled the engraftment rate of the injected stem cell-derived cardiomyocytes. In a research letter in the journalCirculation, co-senior authors Ramaswamy Kannappan, Ph.D., and Jianyi "Jay" Zhang, M.D., Ph.D., say their robust approach to select functionally competent, intact-DNA cells from a heterogeneous population can be easily adopted in clinical settings to yield cells that are better able to repopulate the ischemic myocardium and improve the performance of a failingheart.
Zhang is chair of UAB Biomedical Engineering, a joint department of the UAB School of Medicine and School of Engineering, and holds the T. Michael and Gillian Goodrich Endowed Chair of Engineering Leadership. Kannappan is an assistant professor in the UAB Department of Biomedical Engineering.
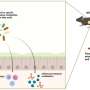
Cardiac cell transplantation requires millions of stem cells or their differentiated derivatives. Cell propagation under accelerated growth conditions is a common way to get these large numbers of cells; but accelerated growth causes culture stress, including lethal DNA damage. These DNA-damaged cells are not suitable for cell transplantation and have to be removed from cell preparations.
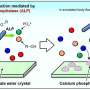
The researchers found they could activate transcription factor p53 in induced pluripotent stem cells to selectively induce programmed cell death, or apoptosis, specifically in DNA-damaged cells, while sparing DNA damage-free cells. They used Nutlin-3a, an MDM2 inhibitor, to activate the p53. After Nutlin-3a treatment, the dead cells were washed from the culture, and the remaining DNA damage-free cells were cultured normally and differentiated into cardiomyocytes.
They then injected 900,000 of the derived cardiomyocytes into the border zone in the left ventricle of the mouseheart-attackmodel. Four weeks later, the researchers found a significantly higher engraftment rate, about 14 percent, in hearts that received the DNA damage-free cardiomyocytes. Engraftment of the control derived cardiomyocytes was about 7 percent.
"To our knowledge," Kannappan and Zhang said, "this is the first study to show that DNA damage-free induced pluripotent stem cells can be selected by p53 activation in induced pluripotent stem cell cultures, and that DNA damage-free cardiomyocytes have enhanced cardiac engraftment potential."
Previous research by others has shown that DNA-damaged senescent cells do not undergo cell death. Instead, they remain within the tissue, with altered functions that can change the tissue microenvironment and promote aging phenotypes of other cells. This may be one explanation for the engraftment advantage of DNA damage-free derived cardiomyocytes.
The method to remove DNA-damaged cells may have wider application, Kannappan says.
"As this is a小moleculebased approach to select DNA damage-free cells," he said, "it can be applied to any type ofstem cells, though selection conditions would need to be optimized and evaluated. Other stem cell approaches for diseases such as neurodegenerative diseases, brain and spinal cord injuries, and diabetes might benefit by adopting our method."
Explore further