Enhancing immune checkpoint inhibitor therapy using treatment combination

A combination of a novel inhibitor of the protein CK2 (Casein kinase 2) and an immune checkpoint inhibitor has dramatically greater antitumor activity than either inhibitor alone, according to research from The Wistar Institute that was published online inCancer Research.
Immunecheckpointinhibitors have been approved to treat several types ofcancer, including some types of lung cancer and colon cancer, but not all patients who receive these immunotherapeutics gain benefit from them. A better understanding of the molecular reasons why some patients do not respond to immune checkpoint inhibitors could identify new therapeutic targets for combination treatments that may improve clinical outcomes.
“免疫细胞称为myeloid-deriv人口ed suppressor cells (MDSC) has been implicated in tumor resistance to various types of cancer treatment, including immune checkpoint inhibitors," said lead researcher Dmitry I. Gabrilovich, M.D., Ph.D., Christopher M. Davis Professor and program leader of the Immunology, Microenvironment & Metastasis Program at Wistar. "We have previously shown that accumulation of the most abundant type of MDSC, polymorphonuclear MDSC (PMN-MDSC), is caused by downregulation of Notch signaling, in part as a result of CK2 activity."
Based on these previous results, Gabrilovich and collaborators set out to investigate whether combining inhibitors of CK2 with immune checkpoint inhibitors could improve immune responses in mouse models of cancer and to determine what mechanisms of action caused the results they obtained.
"Our new data suggest that using a CK2 inhibitor to manipulate the tumor microenvironment may sensitize patients to the effect of an immune checkpoint inhibitor and thereby improve clinical outcomes, although this needs to be tested in clinical trials," said Ayumi Hashimoto, a postdoctoral fellow in the Gabrilovich Lab and first author on the paper.
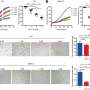
The researchers found that a combination of the CK2 inhibitor BMS-595 and the免疫checkpoint inhibitoranti-CTLA-4-mIgG2a在三种抗肿瘤活性different mouse models of cancer: a lung cancer, a colon cancer, and a lymphoma model. More than 60 percent of mice who received the combination treatment completely eliminated the tumor while none of the mice that received either single agent alone completely eliminated the tumor.
The mechanism of the effect of BMS-595 was analyzed, and in-depth studies showed that two of the main types of immune cells affected by the CK2 inhibitor in tumor-bearing mice were PMN-MDSCs and another type of myeloid cell called tumor-associated macrophages (TAMs). The frequency of PMN-MDSCs was not significantly altered in tumors, but it was substantially decreased in the spleen, an organ that is a part of the immune system. TAMs were decreased in the tumor.
"Our study shows that CK2 inhibition blocks the differentiation of PMN-MDSCs and TAMs, meaning that it blocked the generation of these cells from their precursors. This led to a decrease in immunosuppressive PMN-MDSCs andtumor-promoting TAMs and thus to a substantial augmentation of the antitumor activity of immune checkpoint blockade," added Gabrilovich.
Explore further