Breyanzi approved for certain types of large B-cell lymphoma

细胞基因治疗Breyanzi (lisocabtagene maraleucel) has been approved to treat adults with certain types of large B-cell lymphoma who have not responded to other systemic treatment, the U.S. Food and Drug Administration announced Friday.
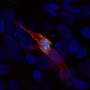
The chimeric antigen receptor T cell therapy was approved for relapsed or refractory large B-cell lymphoma, including diffuse large B-cell lymphoma, high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3. It is not indicated for patients with primary central nervous system lymphoma. The therapy is indicated for patients who have not responded to or have relapsed after two or more other systemic treatments.
The approval was based on safety and efficacy data from TRANSCEND NHL 001, a multicenter clinical trial of 268 patients with refractory or relapsed large B-cell lymphoma. Researchers found that 73 percent of patients achieved a response, including 54 percent who achieved complete remission and 19 percent who achieved a partial response, after treatment with Breyanzi. The median duration of response overall was 16.7 months, and patients who achieved a complete response did not reach a median duration of response. Of 104 patients who achieved complete remission, 65 and 62 percent had complete remission lasting at least six months and at least nine months, respectively.
Breyanzi can causeserious side effects, and its labeling includes a boxed warning for cytokine release syndrome (CRS). Other potential side effects includehypersensitivity reactions, serious infections, low blood cell counts, and a weakened immune system. The FDA notes that side effects typically appear within one to two weeks following treatment, but some may occur later. Because Breyanzi has the potential to cause CRS and neurological toxicities, the FDA approval includes a risk evaluation and mitigation strategy requiringhealth care facilitiesdispensing Breyanzi to be specially certified. The FDA is also requiring the manufacturer to conduct a postmarketing observational study of patients treated with Breyanzi.
The approval was granted to Juno Therapeutics, a Bristol-Myers Squibb Company.
More information:More Information
Copyright © 2020HealthDay. All rights reserved.